

Magnet basics
How do magnets work?
Questions that often come up are, "How do magnets work?", or, "Why is iron magnetic?", or, "What makes a magnet?", or, "What is the magnetic field made of?".
Those are good questions, and deserve a good answer. However, did you know that there is a lot about magnets at the atomic level that isn't known yet? Just like with most of the other basic forces we are familiar with, such as gravity, electricity, mechanics and heat, scientists start by trying to understand how they work, what they do, are there any formulas that can be made to describe (and thus predict) their behavior so we can begin to control them, and so on.
The work always starts by simple observation (that's the fancy word for playing around with the stuff!). That's why it's so important to have some "hands-on" experience with magnets. Have you taken two magnets and tried to push like poles together? How far away do you start to feel the repulsion? How does the force vary with the distance between them? When the magnets are moved off-axis to each other (moving them to the side and not head on) what does it feel like? Could you describe it like trying to push two tennis balls together? When you flip one around, what changes? What about moving one around the other in a circle? Try these things! That's how you learn! Only when you play with (observe) them will you begin to understand how they work. This is the stuff great scientific pioneers did, like Faraday, Lenz, Gilbert, Henry and Fleming.
What we can find out this way, is some of the basics of magnetism, like:
Now, the fun begins. We start to ask the question, "Why?" This is what scientists continually do - try to figure out why things behave the way they do. Once we figure that out, we have a better handle on how to apply them to make useful tools for us, right?
Let me share with you some of what is known about how magnets work. All of the questions have NOT been answered, perhaps you will help answer some of them. So, some of what is known are simply observations, some are guesses, but a lot has been figured out.
There are only a few elements in the periodic table that are attracted to magnets. None of the elements, by themselves, make good permanent magnets, but can become temporary magnets (when close to another magnet). When alloys of various metals are made, some of these alloys make very good magnets. Why? We don't really know, but we can observe some consistent rules.
As you know, we have seen that when current flows in a wire, a magnetic field is created around the wire. Current is simply a bunch of moving electrons, and moving electrons make a magnetic field. This is how electromagnets are made to work. This will be important to keep in mind as we zoom into the structure of atoms.
Around the nucleus of the atom, where the protons and neutrons live, there are electrons whizzing around. We used to think that they had certain circular orbits like the planets have around the sun, but have discovered that it is much more complicated, and much more exciting! Instead, the patterns of where we would likely find the electron within one of these orbitals takes into account Schroedinger's wave equations. Pictures of each of these orbitals can be found at http://www.shef.ac.uk/chemistry/orbitron/index.html. (These also take into account Heisenberg's uncertainty principle and probability theory.)
First, electrons can be thought of as occupying certain shells that surround the nucleus of the atom. These shells have been given letter names like K,L,M,N,O,P,Q. They have also been given number names, such as 1,2,3,4,5,6,7. (This is what quantum mechanics is all about).
Within the shell, there may exist subshells or orbitals, with letter names such as s,p,d,f. Some of these orbitals look like spheres, some look like an hourglass, others look like beads on a bracelet.
The K shell contains an s orbital. Called a 1s orbital.
The L shell contains an s and p orbital. Called a 2s and 2p orbital.
The M shell contains an s, p and d orbital. Called a 3s, 3p and 3d
orbital.
The N, O, P and Q shells each contain an s, p, d and f orbital. Called a
4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d, 6f, 7s, 7p, 7d and 7f orbital.
These orbitals also have various sub-orbitals.
The s orbital can contain only 2 electrons and has no sub-orbitals.
The p orbital can contain 6 electrons, 2 in each of its 3 sub-orbitals,
like px, py and pz.
The d orbital can contain 10 electrons, 2 in each of its 5 sub-orbitals, like
dxy, dxz, dyz, dz2, dx2-y2.
The f orbital can contain 14 electrons, 2 in each of its 7 sub-orbitals.
(And there is a g orbital that can contain 18 electrons, 2 in each of its 9
sub-orbitals, for highly excited electrons.)
A maximum of 2 electrons can occupy a sub-orbital where one has a spin of UP, the other has a spin of DOWN. There can not be two electrons with spin UP in the same sub-orbital. (Pauli exclusion principal.) Also, when you have a pair of electrons in a sub-orbital, their combined magnetic fields will cancel each other out.
In order to show how many electrons are in each orbital, the following
convention is sometimes used:
Chlorine
has 1s22s22p63s23p5
for a total of 17 electrons. This tells us that there are 2 in 1s, 2 in
2s, 6 in 2p, 2 in 3s, and 5 in 3p.
Let's look at the pattern of how the electron orbitals are filled as we move up in the periodic table of the elements. (This is a fantastic site on the elements!!!)
[DIRECTIONS: click on the figure above to go to http://www.webelements.com/webelements/elements/text/Fe/econ.html. Find the box on that page like the one above. Click on the left and right arrow buttons within that box to see how the electron orbitals and sub-orbitals are filled as you step through the periodic table.]
As you can see, the general order for filling the electron orbitals follows
a sequence since the energy level for each orbital increases in this sequence:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
After each orbital is full, it starts to fill the next one in this sequence. There are a few odd jumps in the sequence when you get to filling the 4f, 5d and 6p orbitals, but that's how it goes.
If we were to examine Iron (atomic number 26), Cobalt (27), Nickel (28) and Gadolinium (64), all of which are considered ferromagnetic since they are strongly attracted to a magnet, it is difficult to see what makes them so different from the other elements next to them or below them in the periodic table. In other words, if Iron is so strongly magnetic, why isn't Manganese? Perhaps there are other factors we need to take into account such as the crystalline structure. But it is generally accepted that these ferromagnetic elements have large magnetic moments due to un-paired electrons in their outer orbitals. This is like having current flowing in a coil of wire, creating a magnetic field. Even the spin of the electron is thought to create a minute magnetic field. When you get a bunch of these fields together, they add up to bigger fields.
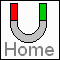
Iron (Fe)
Atomic Number 26
Electron configuration 1s22s22p63s23p63d64s2
This shows the electron orbits as circular rings around the nucleus. It
really isn't like this, but it makes a good diagram.
The green dot in the center is the nucleus with the 26 protons and 26
neutrons.
The orange dots in the 3d orbital are the 4 unpaired electrons.
The unpaired electrons in 3d create a magnetic moment, or force. It is
thought that D/r must be 3 or more to create ferromagnetism. This
condition occurs in Iron, Cobalt, Nickel and rare-earth groups.
We can go one level deeper into quantum mechanics. This is where we ask, "What is the magnetic field made of?"
Today, there are four basic forces that are known: gravity, electromagnetism, weak, strong. What creates these forces? There is speculation among particle physicists that these forces are the result of photons that are exchanged between particles. This exchange is what creates a repulsion or attraction between various particles, giving us the forces we call gravity, magnetism, and others that hold the protons together in the center of the atom. More information on these Virtual Particles is available at this link.
For a more in-depth understanding, you will
want to read about the Standard Model of the atom at
http://www.particleadventure.org/standard_model.html
and
http://particleadventure.org/
1. Magnetic moments in neighboring atoms are held parallel by quantum mechanical forces.
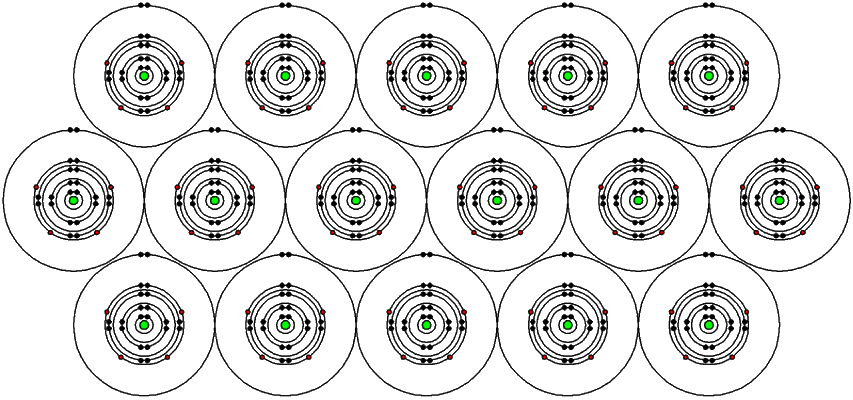
2. These atoms with these magnetic characteristics are grouped into regions called domains. Each domain has its own North pole and South pole.
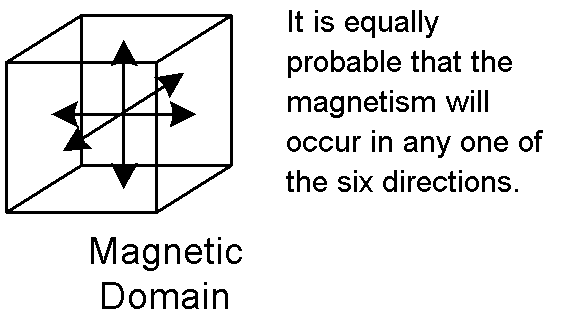
A Domain is the smallest known permanent magnet. About 6000 domains
would occupy an area the size of the head of a common pin.
A domain is composed of approximately one quadrillion (1,000,000,000,000,000
or 1015) atoms.
3. In unmagnetized ferromagnetic materials, the domains are randomly oriented and neutralize each other or cancel each other out. However, the magnetic fields are still present within the domains!
(These diagrams show domains as small cubes or squares - kind of a micro view.)
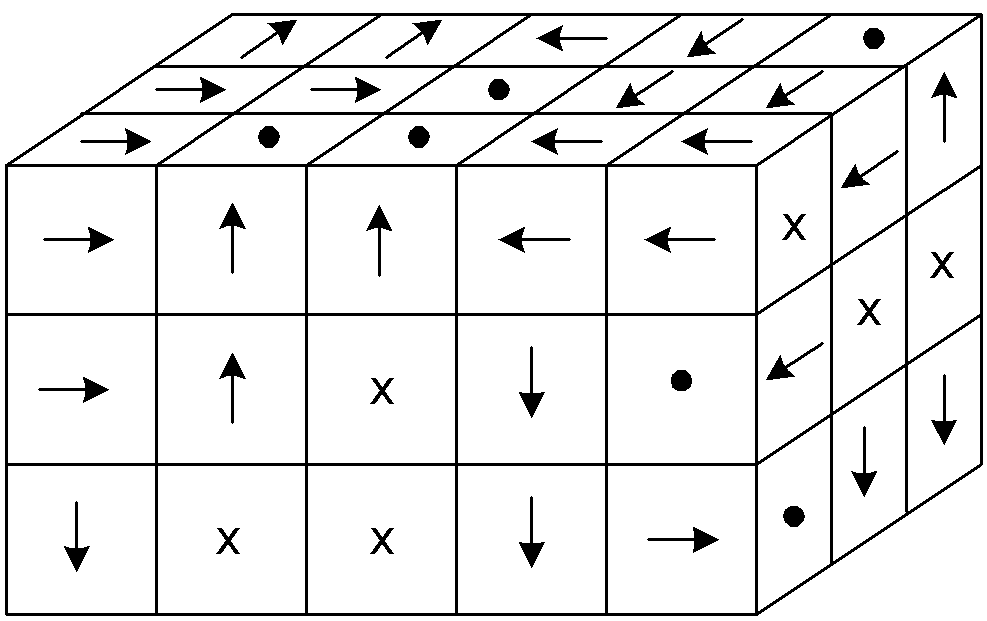
Here is a sample of unmagnetized iron, showing its domains in random magnetic
orientations (x is arrow away from you = South Pole, dot is arrow toward you =
North Pole)
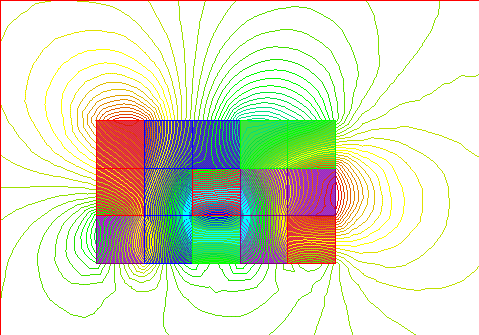
This shows the magnetic field around that sample of unmagnetized iron with its
groups of domains, like those noted above, with random orientations. As
you can see, this sample has multiple North and South poles where the magnetic
field lines exit and enter the material.
4. The application of an external magnetic field causes the magnetism in the domains to become aligned so that their magnetic moments are added to each other and lined up with the applied field.
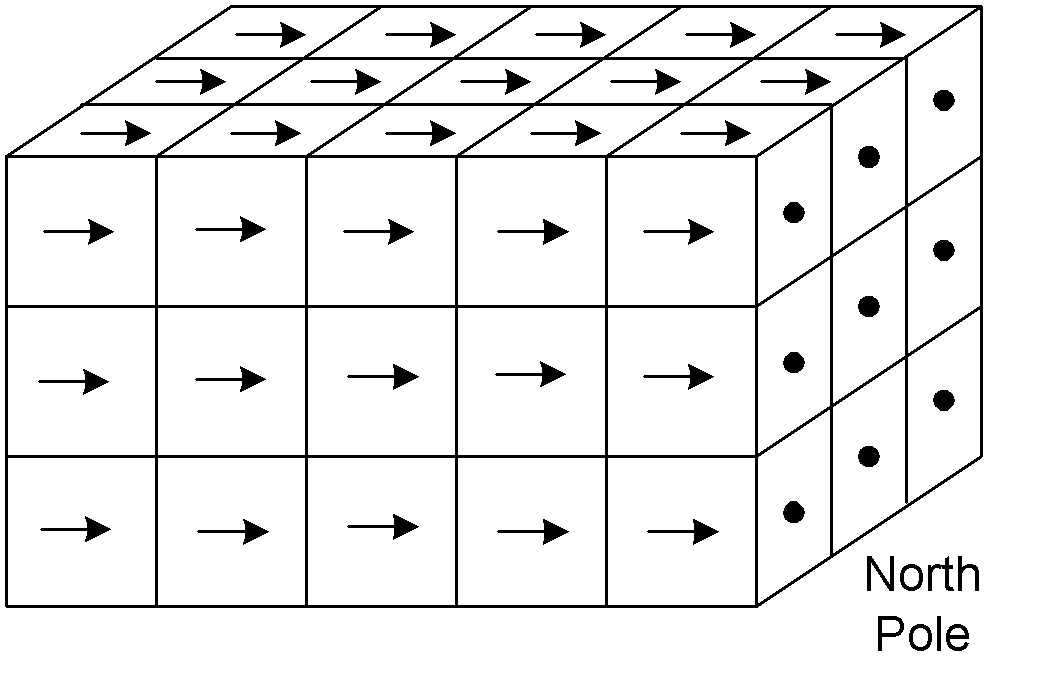
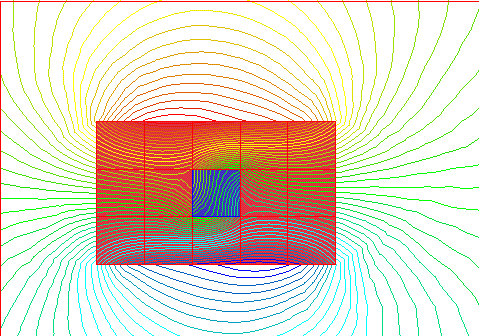
This shows the magnetic field around a group of domains, where all but one is
oriented in the same direction.
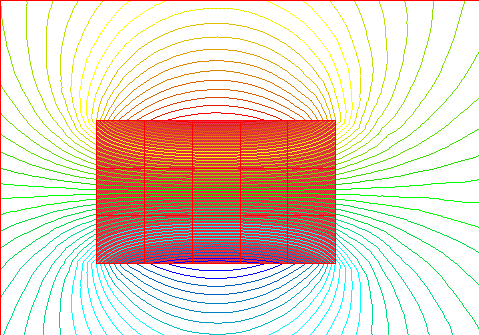
And this shows the magnetic field around a group of domains that are all lined
up together.
With soft magnetic materials such as iron, small external fields will cause a great amount of alignment. However, because of the small restraining force only a little of the alignment will be retained when the external field is removed.
With hard magnetic materials such as Alnico a greater external field must be applied to cause alignment of the domains, but most of the alignment will be retained when the field is removed, thus creating a stronger permanent magnet, which will have one North and one South pole.
If we were to look at this from more of a macro level, a level at which we have actually seen under microscopes, we would see larger domains - not as cubes or squares, but more like irregular polygons.
If you were to examine a piece of iron that is not magnetized, you will find that the domains within the iron will not be pointing in the same direction, but will be pointing in a bunch of random directions. This randomness is what causes the magnetic field of each domain to be cancelled out by the magnetic field of another domain. The result is that there is no single north pole or south pole. Instead, there are a bunch of north and south poles all over the place that cancel each other out.
Now, if this piece of iron were placed within an external magnetic field (created by current flowing in a solenoid), the domains will start to line up with the external magnetic field. It takes some energy to cause a domain to re-orient itself. As the external magnetic field becomes stronger, more and more of the domains will line up with it. (Another way to look at it is that the domains that are aligned with the external magnetic field will grow in size, and the others will shrink.) There will be a condition where all of the domains within the iron are aligned with the external magnetic field. This condition is called saturation, because there are no more domains that can be lined up, no matter how much stronger the magnetic field is made.
(These diagrams show domains as irregular polygons - more of a macro view.)
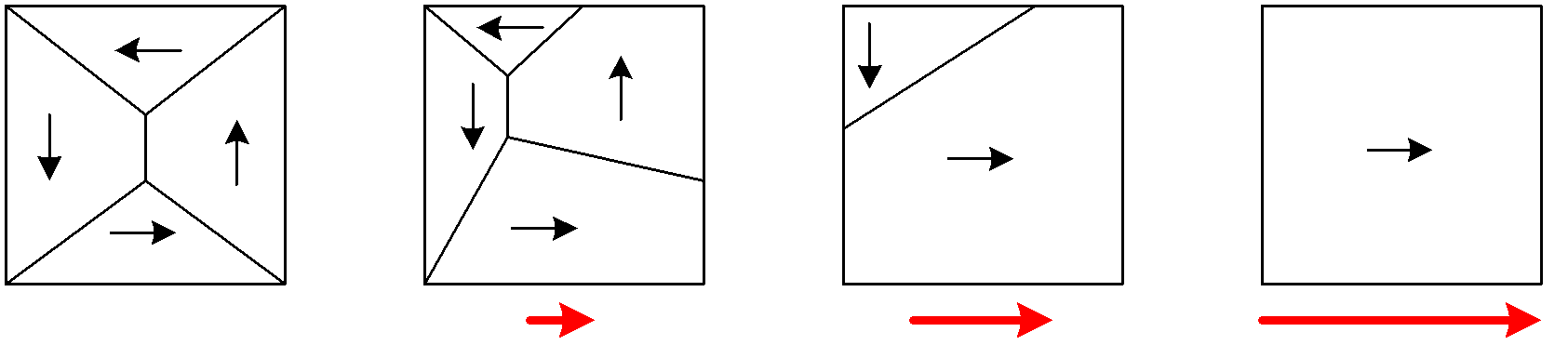
no external mag
field small
mag
field
larger mag
field
large mag field, saturation of domains
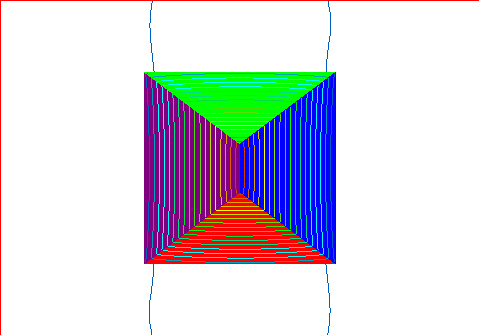
Resulting magnetic field with the domains as indicated above with no external
mag field.
Note that the domains still have their own magnetic field, but that the field
lines stay almost exclusively within the material.
Very little leaks out of the material. This would be an example of
unmagnetized iron.
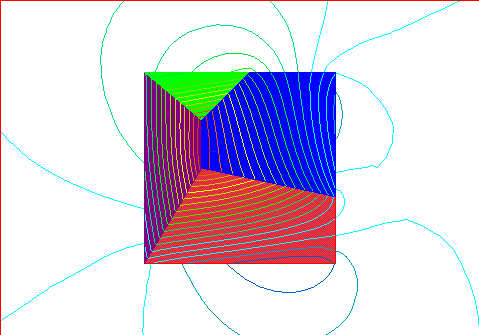
Resulting magnetic field with the domains as indicated above with small mag
field.
This has two north poles (lower right and upper right) and one very spread-out
south pole (on the left).
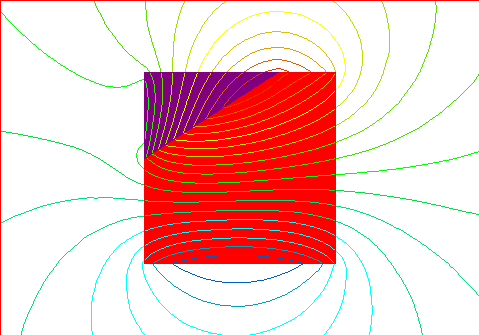
Resulting magnetic field with the domains as indicated above with larger mag
field.
Starting to look more like a magnet with a defined north and south pole.
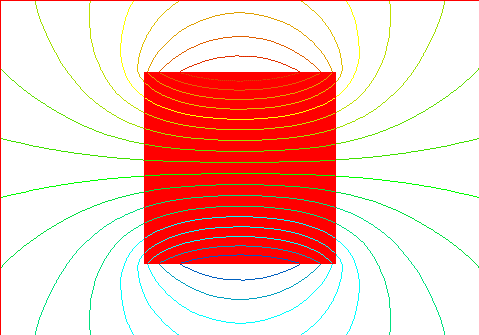
Resulting magnetic field with the domains as indicated above with large mag
field, saturation of domains.
This is what a permanent or temporary magnet would typically look like.
What happens when the external magnetic field is reduced back to zero? In a soft magnetic material (such as iron or silicon steel), most of the domains will return to their random orientations, so that you will be left with a very weak magnet since only a few of the domains will be lined up in the same direction. In other words, you are back where you started from. In a hard magnetic material (alloys of iron such as Alnico, some steels, neodymium-iron-boron, etc), most of the domains will remain aligned, so that you will be left with a strong magnet. Since the ending point is not the same as the starting point for magnetic materials, they have what is called hysteresis.
1. A freely suspended bar magnet will always tend to align itself with the North and South magnetic poles of the earth. An example of this is the magnetic compass.
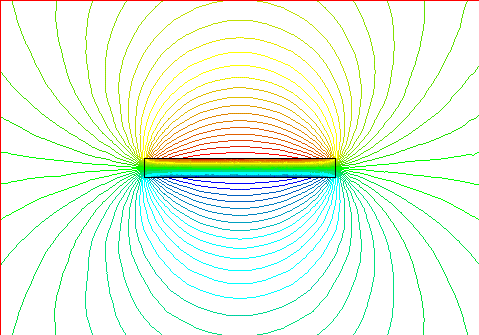
This shows the magnetic lines of force for a long, narrow bar magnet.
North is on the right end.
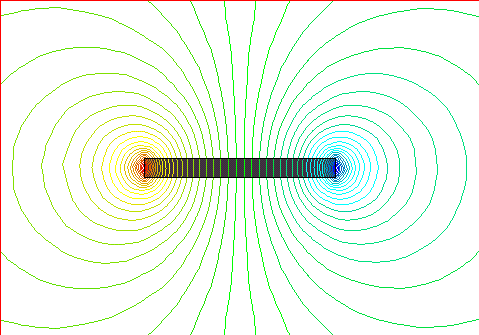
This shows the magnetic lines of force for a flat, wide magnet. North is
on top.
Note the concentration of lines where they exit or enter the magnet, at the ends. This is what defines a pole. Since magnetic fields are like rubber-bands, and since they like to crowd into ferromagnetic material whenever they can, they bunch up inside the magnet material. Again, since the field lines are like closed loops, there is always some place where they enter the magnet (South pole), and some place where they exit the magnet (North pole). These places are the poles. The magnetic field lines tend to be closest together there. This is why, if you break a magnet in half, you will still have a North pole and a South pole, since the lines enter one magnet, then exit it, then enter the next magnet, then exit it, before it goes back to the first magnet again. This is also why we can't have a "monopole" or single pole. If the magnetic field line exits the magnet, somewhere it will have to enter it again - the loops are closed like rubber-bands. The minimum number of poles a magnet can have is two - one each of North and South. However, it is possible for a magnet to have more than two poles, right? Look at the pictures above again, where we have a lot of random square domains. See all the poles all around the periphery of the group of domains? I count about 10! Below is a magnet with 8 poles.
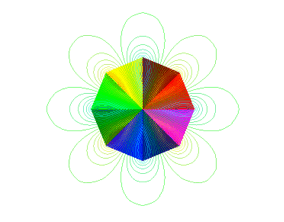
This magnet has 8 poles - 4 North and 4 South, or 4 pole-pairs.
A compass in the vicinity of a magnet will always point along a tangent to one
of the magnetic field lines.
This occurs because unlike poles of a magnet are always attracted to each other by the invisible lines of force whereas like poles repel each other. The earth acts like a large permanent magnet. In fact, the earth is the largest magnet in the world. But don't forget the sun that also has a magnetic core, and so do collapsed stars, and they are bigger than the earth! I wouldn't call the earth a permanent magnet like other magnets we are used to. Its magnetism is the result of electron convection currents in the liquid core, and they have flipped around a few times in the past, just like what the sun does every 11 years. So, it's really more like an electromagnet.
2. Permanent magnets can be designed and engineered in hundreds of shapes and sizes to perform various tasks.
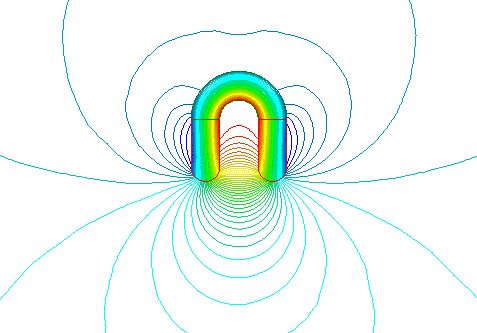
For example, the horse-shoe shape is very commonly used in magnetic separators because its lines of force are mostly at the open end of the horse-shoe, and this helps in the separation of ferrous materials. A piece of iron placed within the effective range of the magnetic field will, in turn become magnetized. It will have its own North and South poles and will be attracted to the permanent magnet.
What all this is saying, is that the atoms of ferromagnetic materials tend to have their own magnetic field created by the electrons that orbit it.
Small groups of about 1015 atoms tend to orient themselves in the same direction. These groups are called domains. Each domain has its own north pole and south pole.
If you were to examine a piece of iron that is not magnetized, you will find that the domains within the iron will not be pointing in the same direction, but will be pointing in a bunch of random directions. This randomness is what causes the magnetic field of each domain to be cancelled out by the magnetic field of another domain. The result is that there is no single north pole or south pole. Instead, there are a bunch of north and south poles all over the place that cancel each other out.
Now, if this piece of iron were placed within an external magnetic field (created by current flowing in a solenoid), the domains will start to line up with the external magnetic field. It takes some energy to cause a domain to re-orient itself. As the external magnetic field becomes stronger, more and more of the domains will line up with it. (Another way to look at it is that the domains that are aligned with the external magnetic field will grow in size, and the others will shrink.) There will be a condition where all of the domains within the iron are aligned with the external magnetic field. This condition is called saturation, because there are no more domains that can be lined up, no matter how much stronger the magnetic field is made.
When the external magnetic field is then removed, soft magnetic materials will become randomly oriented domains again. However, hard magnetic materials will keep most of their domains aligned, making it a strong permanent magnet.
Magnetic field lines are closed loops. They enter a magnet at its South pole, and exit a magnet at its North pole. The poles may cover a large area, where the concentration of lines is not uniform.
Did you notice all of the scientific disciplines that are involved with magnets? I'll list the ones I can think of, perhaps you can add some to this list.
quantum mechanics
probability theory
particle physics
chemistry
mathematics
material science
electrical engineering
application engineering
those-who-like-to-play-around-with-magnets engineering
NOTE:
Some of the material shared above was originally presented by Arlo F.
Israelson, Chief Engineer at Eriez Manufacturing Co., in Erie, Pennsylvania,
USA, dated 9/12/52. This was found in Permanent Magnet Design and
Application Handbook, by Lester Moskowitz, Cahners Books International,
Boston, MASS 1976, in Chapter 6, Fundamentals of Magnetism. The
illustrations and additional notes are mine, patterned after his presentation.